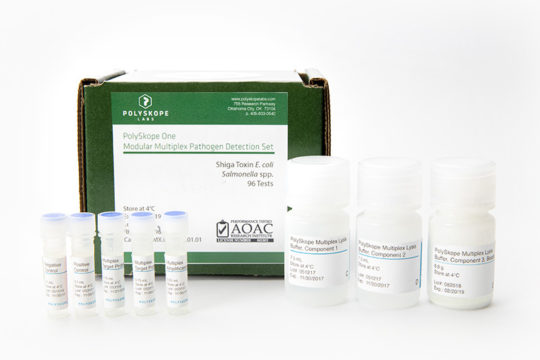
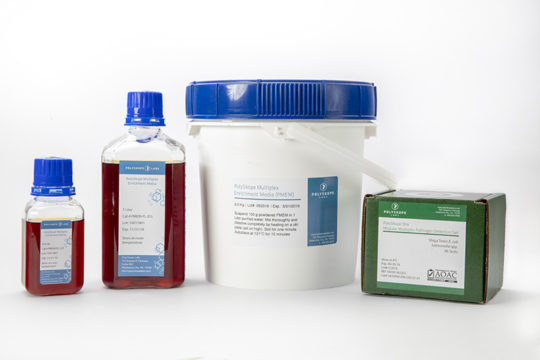
PolySkope One allows you to simplify your microbial contaminant testing for E. coli & Salmonella spp. by switching to a single multiplex qPCR kit & all-in-one enrichment media.
PolySkope One is the first ever AOAC-approved true multiplex test kit & media for the simultaneous detection of any or all common/routine foodborne pathogens including: E. coli O157:H7, Shiga Toxin E. coli, Salmonella spp., and Listeria monocytogenes (Listeria spp. target set available). PolySkope is designed to simplify and reduce the cost of pathogen testing using our revolutionary novel enrichment medium (PMEM) combined with tried-and-true qPCR chemistry, a proven commodity in clinical, cannabis, and food safety testing. PolySkope One is a flexible and modular multiplex; routine pathogens are detected individually or simultaneously (user selectable) with one enrichment and protocol.
All other approved pathogen-testing kits require unique and separate enrichment media, different incubation temperatures, and unique plastics & reagents. The PolySkope One multiplex simplifies workflow and reduces the material & non-material cost of testing. This reduces: training, labor, SOP’s & regulatory burden, lab & storage footprint, error rate, plastics, energy consumption, and logistics & inventory complexity.
PolySkope One also reduces the environmental impact of testing significantly by using less media, plastics, water and energy (incubators, reagent cold storage, instrument run, etc.)